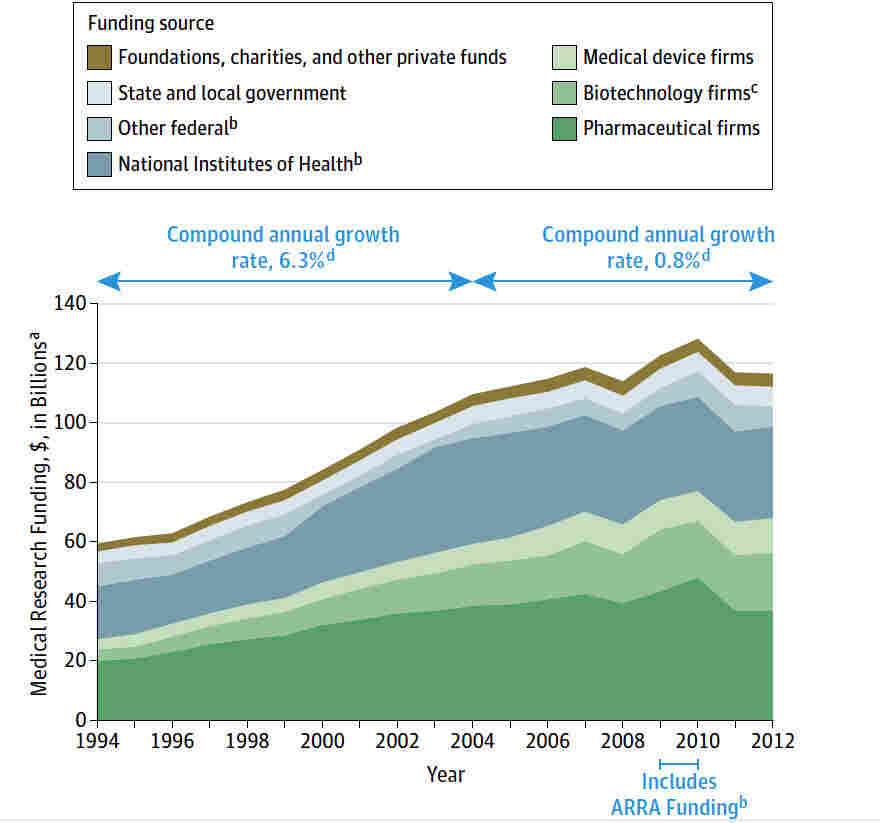
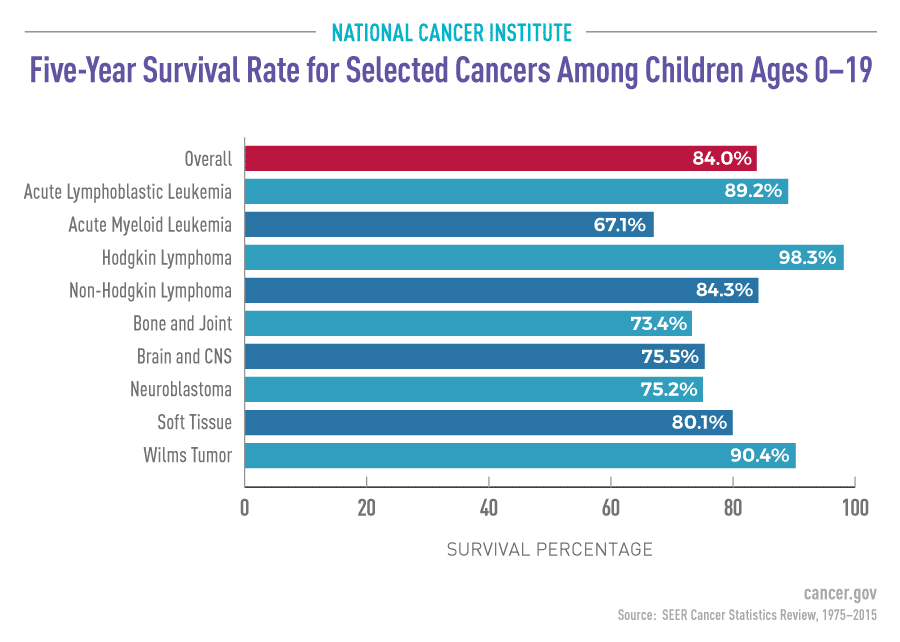
Medical research funding plays a crucial role in advancing healthcare and ensuring patient safety in research. However, recent funding cuts have raised significant concerns about the future of clinical studies and the ethical oversight required to protect participants. With reduced financial resources, the capacity of Institutional Review Boards (IRBs) to monitor adherence to clinical research ethics is severely compromised, jeopardizing patient rights and welfare. Moreover, the impacts of these funding cuts extend to the overall quality of research, as less support can lead to increased skepticism about the validity of findings and the safety of new treatments. Understanding the effects of diminishing NIH funding is essential to grasp how these financial decisions shape public trust in medical research and its ultimate benefit to society.
Securing financial support for scientific inquiry into health is vital for the progression of effective treatments and patient care. The decline in available grants not only threatens the advancement of knowledge but also raises alarms regarding the ethical management of research projects. This situation highlights the importance of maintaining rigorous oversight through Institutional Review Boards (IRBs) to ensure that human subjects are safeguarded throughout their participation in trials. The ethical dimensions of conducting medical studies cannot be overstated, as funding intricately ties to the integrity of the research process itself. As discussions about the ramifications of reduced funding grow, the implications for clinical research and patient safety are increasingly scrutinized, emphasizing the need for renewed commitment to supporting this essential work.
The Consequences of Funding Cuts on Medical Research Safety
The recent cuts to medical research funding pose an undeniable threat to the intricate system designed to secure patient safety during clinical trials. With a halt in over $2 billion in federal research grants to institutions like Harvard, the operational continuity of crucial oversight mechanisms, such as those provided by Institutional Review Boards (IRBs), is severely compromised. These boards play a fundamental role in protecting human participants from potential risks associated with new treatments or medical interventions. With research activities disrupted, existing safeguards are weakened, increasing the likelihood that ethical standards may not be upheld. This tumultuous scenario sheds light on how funding cuts not only impact research capabilities but ultimately affect the safety and rights of patients involved in studies.
Furthermore, the lack of adequate funding leads to a ripple effect, resulting in delays and potential ethical oversights within ongoing research projects. For example, when funding cuts halt the collaboration between various research sites, as seen with the SMART IRB’s stop-work order, it impedes the ability to review and approve new clinical trials effectively. This stagnation could lead to scenarios where emerging treatments are not vetted properly, thereby compromising patient safety in medical research. The urgency of securing appropriate funding cannot be overstated, as maintaining operational integrity is crucial for fostering trust in the research process and ensuring that patients’ interests remain a priority.
NIH Funding Effects on Patient Safety and Research Oversight
National Institutes of Health (NIH) funding plays a pivotal role in bolstering patient safety through comprehensive oversight mechanisms in clinical research. NIH policies mandate that research involving human subjects is thoroughly scrutinized by Institutional Review Boards (IRBs), which are essential for ensuring that the rights and welfare of study participants are rigorously protected. Without adequate NIH funding, many institutions might struggle to fulfill these extensive safety requirements, leading to a potential degradation in ethical standards across several research initiatives. The implications of insufficient funding can extend well beyond immediate operational constraints, negatively affecting the trust that participants place in medical research.
Moreover, NIH funding effectively underpins the collaborative nature of multisite research, which is critical in today’s complex medical landscape. With the introduction of single IRB reviews for multisite studies, the expectation is that NIH will facilitate streamlined oversight that ensures patient protections remain intact. However, any reduction in this funding compromises the resources necessary for conducting thorough reviews and ensuring compliance with best practices in clinical research ethics. When funding is at stake, the risk of overlooking patient safety increases, potentially jeopardizing both the validity of research outcomes and the well-being of those who volunteer to participate.
Understanding the Role of IRBs in Clinical Research Ethics
Institutional Review Boards (IRBs) serve as a critical mechanism in the ethical oversight of medical research involving human participants. They evaluate research proposals with a unique lens focused on participant safety, informed consent, risk assessment, and the ethical considerations that arise during studies. The historical foundations of IRBs were built upon significant ethical breaches in research practices, leading to stringent regulations that protect participants’ rights and welfare. In recent discussions, the transparency and accountability of IRBs have been emphasized, particularly in light of calls for reforms aimed at enhancing patient safety in research.
The function of IRBs extends beyond mere compliance; they actively engage researchers in discussions surrounding ethical obligations and participant rights. Their role becomes even more crucial in the current climate of funding cuts, where they must be vigilant in maintaining high standards of oversight despite potential reductions in their operational capabilities. The reliance on robust IRB oversight is paramount, particularly when the funding environment for medical research is strained. The ongoing dialogue about clinical research ethics must prioritize ensuring that IRBs are adequately supported so that they can continue to fulfill their essential role in safeguarding participants.
Restoring Trust in Medical Research Amid Funding Challenges
The challenges faced by medical research funding can significantly affect the public’s perception of clinical trials and academic institutions. Trust is integral to the volunteer participation necessary for the advancement of medical knowledge, and any disruption in research funding can erode that trust. The halting of numerous studies due to funding cuts has not only delayed potential breakthroughs in patient care but also raised concerns about the ethical oversight and the conduct of research. With historical precedents demonstrating the ramifications of unethical research practices, such as the Tuskegee syphilis study, the need for stringent and effective IRB oversight is more pressing than ever.
In order to restore trust, institutions must convey transparency about the funding challenges and actively communicate how they are addressing concerns related to patient safety in research. This involves re-evaluating governance structures and ensuring that ethical oversight remains a priority despite funding constraints. Engagement with community stakeholders and research participants is crucial for rebuilding confidence. By working collaboratively and transparently, institutions can reaffirm their commitment to ethical research practices and demonstrate that, even in challenging funding environments, patient safety and rights remain central to the research mission.
The Impact of Federal Funding on Research Ethics and Oversight
Federal funding is essential not only for advancing scientific discovery but also for enforcing the ethical standards that govern this research. Without the influx of financial support from agencies such as the NIH, institutions may find it challenging to uphold the integrity of their research programs. Funding is allocated not only for project execution but also for the infrastructure that supports compliance with ethical standards and oversight protocols. Consequently, any reduction in federal funding could lead to a decline in the rigorous evaluation and oversight needed to safeguard patient safety in research.
Moreover, the ethical landscape of clinical research is evolving. As funding paradigms shift, there must be a concurrent emphasis on adapting oversight frameworks to sustain ethical integrity. Researchers must be educated on the implications of funding cuts, and institutions need to prepare for how these changes could impact their IRB processes and patient protection measures. Keeping the dialogue open about the interdependencies of funding, research ethics, and participant safety will remain critical as the research community navigates the complexities of the current funding climate.
Challenges for Collaborative Research in a Funding-Constrained Environment
Collaborative research is increasingly recognized as a vital component of medical innovation, enabling various institutions to pool resources and knowledge in pursuit of cutting-edge advancements. However, funding cuts pose significant challenges to these collaborative efforts. As noted in recent reports, disruptions to shared funding mechanisms like SMART IRB can severely hinder the ability for diverse institutions to engage in joint research initiatives. Consequently, the potential for breakthroughs in patient care is compromised, as multidisciplinary collaborations often lead to more comprehensive and effective research outcomes.
Aside from delaying new studies from launching, the cancellation of research funding can also perpetuate a cycle of mistrust from the public toward research institutions. Communities involved in collaborative projects may feel undervalued when their engagement becomes transactional or halts due to financial limitations. This loss of confidence could discourage participation in clinical trials, which are essential for testing new treatments. To mitigate these challenges, it is essential for researchers to communicate the value of collaborative activities clearly and to advocate for sustained funding that prioritizes partnership-based approaches to clinical research.
Revolutionizing Patient Safety through Increased Funding
In the current landscape of medical research, increasing funding presents an opportunity to revolutionize patient safety measures across clinical trials. Essentially, more substantial financial resources allow for the implementation of advanced monitoring technologies and enhanced personnel training, ensuring that participants are not only protected legally but also benefitting from cutting-edge research practices. By allocating funds specifically for patient safety initiatives, institutions can work towards developing transparent systems that address patient concerns and improve overall trust in clinical research.
Investing in patient safety is critical to guiding the future of ethical research. The integration of technology in monitoring patient responses and outcomes during clinical trials represents a vital step forward. This innovation is often contingent on the availability of adequate funding. As funding agencies prioritize these advancements, they can help create and sustain environments where patient welfare is placed at the forefront of medical research, resulting in more ethical, dependable, and effective outcomes for all participants.
Upholding Community Trust through Ethical Research Funding
As funding cuts begin to threaten the careful balance of ethical oversight in medical research, the importance of maintaining community trust becomes more pronounced. Research institutions must not only acknowledge the implications of funding limitations but also actively seek ways to involve community stakeholders in the dialogue surrounding clinical trials. This initiative can ensure that diverse voices are considered in research efforts and that ethical practices align with community expectations. By prioritizing transparency and engaging patients in decision-making processes, institutions lay the groundwork for rebuilding trust that is vital for successful research endeavors.
To effectively uphold community trust, researchers and funding bodies must strive to demonstrate their commitment to the ethical dimensions of clinical research. This involves a clear, collaborative approach that highlights both regulatory compliance and compassionate patient interactions. Emphasizing the importance of informed consent and engagement with participants will foster healthier relationships between research entities and the communities they serve. Successfully doing so will not only mitigate the negative impacts of funding cuts but also bolster public confidence in the medical research process.
Frequently Asked Questions
What is the impact of funding cuts on patient safety in medical research?
Funding cuts significantly undermine patient safety in medical research by disrupting oversight mechanisms like Institutional Review Boards (IRBs). Without adequate NIH funding, research studies can be halted, leading to risks of harm to participants, increased mistrust in medical research, and hindered ethical compliance.
How does NIH funding affect the ethical oversight of clinical research?
NIH funding supports the operation of IRBs, which play a crucial role in ensuring ethical oversight of clinical research. These boards review research proposals to safeguard participant rights and welfare, thus fostering a climate of trust and compliance in medical research activities.
In what ways does IRB oversight contribute to the integrity of medical research funding?
IRB oversight ensures that all medical research funded by federal grants adheres to ethical standards, protecting participants from harm. This oversight helps maintain the integrity of the research process, allowing studies to proceed efficiently and responsibly.
What are the consequences of halted medical research funding on ongoing studies?
When funding for medical research is halted, ongoing studies face significant interruptions, which can jeopardize participant safety and lead to loss of valuable data. Additionally, research teams may struggle to secure new collaborators, resulting in delays that can stifle innovation.
How do funding cuts hinder the effectiveness of clinical research ethics?
Funding cuts can cripple the framework supporting clinical research ethics by reducing the resources available for training and supporting IRB members. This compromises their ability to monitor and enforce ethical standards, ultimately risking participant safety and the credibility of research.
What are the ramifications of reduced medical research funding on public trust?
Reduced funding for medical research can increase public skepticism and distrust towards research endeavors. This erosion of trust may discourage participation in studies, which is vital for advancing healthcare and developing new therapies.
How does the SMART IRB program facilitate better patient safety amid funding challenges?
The SMART IRB program streamlines the ethical review process for multi-site studies, enhancing collaboration and efficiency in medical research. Despite funding cuts, this program aims to maintain patient safety by ensuring consistent ethical standards across participating institutions.
What steps can be taken to address the challenges posed by funding cuts to patient safety in medical research?
To address these challenges, stakeholders can advocate for sustained NIH funding, enhance public awareness about the importance of ethical research oversight, and implement alternative funding models that ensure ongoing support for patient safety initiatives in clinical research.
| Key Point | Details |
|---|---|
| Federal Research Grant Freeze | The Trump administration’s freeze of over $2 billion in research grants disrupted patient safety initiatives. |
| Impact on IRB Oversight | The halt affects the SMART IRB system, crucial for overseeing multi-site medical research. |
| IRBs’ Role | IRBs ensure compliance with laws and protect the rights and welfare of research participants. |
| Negative Effects of Funding Cuts | Cuts to funding jeopardize the safety protocols, extend the timeline for research participation, and erode public trust. |
Summary
Medical research funding is critical for maintaining patient safety standards and ensuring the ethical conduct of clinical studies. With recent significant funding cuts, the operational capacity of Institutional Review Boards (IRBs) is notably compromised, affecting oversight and protection of research participants. The responsible management of research practices and adherence to ethical guidelines, bolstered by consistent funding, are essential to foster public trust in the medical research system. It is imperative that we advocate for robust medical research funding to safeguard the future of patient safety and well-being.