Microglial research is at the forefront of understanding the brain’s immune response, particularly in the context of Alzheimer’s disease and other neurodegenerative diseases. These specialized cells act as guardians of the brain, constantly monitoring for signs of injury and illness to ensure optimal brain health. Recent scientific discoveries in neuroscience have revealed that while microglia are essential for pruning synapses and clearing debris, their malfunction can lead to detrimental effects such as accelerated cognitive decline in Alzheimer’s patients. As researchers like Beth Stevens delve into the intricate workings of these cells, they are uncovering novel biomarkers for Alzheimer’s that could revolutionize diagnosis and treatment. This critical area of study not only promises deeper insights into brain health but also paves the way for innovative therapies aimed at improving the lives of millions affected by neurodegenerative disorders.
Investigation into the role of microglial cells—often referred to as the brain’s immune system—has opened new pathways for addressing various cognitive disorders. These immune cells are vital in maintaining neurological balance, and disruptions in their function are closely linked to conditions such as Alzheimer’s and Huntington’s diseases. By exploring this nuanced landscape, researchers are identifying unique indicators that could serve as potential biomarkers for Alzheimer’s, guiding future therapeutic strategies. Understanding how these cells navigate their roles may lead to groundbreaking interventions in neurodegenerative diseases, showcasing the critical intersection of immunology and neuroscience. As this field evolves, the potential to uncover innovative treatments remains a compelling frontier.
The Role of Microglial Research in Alzheimer’s Disease
Microglial research has emerged as a pivotal area of study in understanding Alzheimer’s disease, a leading neurodegenerative disorder affecting millions globally. These specialized brain immune cells are critical in maintaining brain health by regulating synaptic pruning and responding to neural injury. However, their dysfunction can lead to harmful consequences, including the exacerbation of Alzheimer’s pathology. Recent findings suggest that abnormal microglial activity might accelerate the progression of neurodegenerative diseases, making it essential to investigate this pathway further.
By delving into the realm of microglial research, scientists like Beth Stevens are uncovering the intricate mechanisms that connect the brain’s immune responses to cognitive decline. Such research not only enhances our understanding of Alzheimer’s disease but also paves the way for developing new biomarkers that could signal early changes in brain health. These discoveries can lead to targeted interventions that might alter the course of the disease, highlighting the importance of prioritizing microglial studies in the broader context of neurodegenerative disease research.
Exploring Biomarkers for Alzheimer’s Disease
The identification of reliable biomarkers for Alzheimer’s disease is a critical focus in neuroscience research. Biomarkers can provide invaluable insights into the disease’s progression and help clinicians make informed decisions regarding diagnosis and treatment strategies. Recent advancements in neuroimaging and molecular biology have enabled researchers to pinpoint specific biological indicators associated with the onset and progression of Alzheimer’s. These markers could potentially allow for earlier interventions and personalized treatment plans, significantly transforming patient care.
As researchers delve deeper into the connection between biomarkers and Alzheimer’s, the potential for discovering predictive markers that signal the risk of developing the disease increases. Such scientific discoveries also open avenues for therapeutic strategies aimed at modifying disease progression. Scientists are harnessing data from diverse studies, including microglial research, to explore how inflammation and immune mechanisms correlate with biomarker expression, thereby providing comprehensive insights into Alzheimer’s pathology.
Impact of Basic Science on Neurodegenerative Disease Research
The process of scientific discovery in neurodegenerative diseases often begins with fundamental research. In the case of Alzheimer’s disease, basic science has uncovered the role of microglial cells in the brain’s immune system. This foundational understanding is essential, as it lays the groundwork for more targeted research that can lead to breakthroughs in treatment. Investigators like Beth Stevens emphasize that curiosity-driven research not only fuels scientific inquiry but also results in significant health advancements.
Moreover, core research efforts funded by institutions like the National Institutes of Health (NIH) facilitate long-term studies that can illuminate complex disease mechanisms. These initiatives have shown that even seemingly unrelated studies can converge to reveal vital insights into neurodegenerative diseases. As researchers explore connections between basic cell functions and their implications for diseases like Alzheimer’s, they are carving paths toward innovative therapies that harness the brain’s own resistance mechanisms.
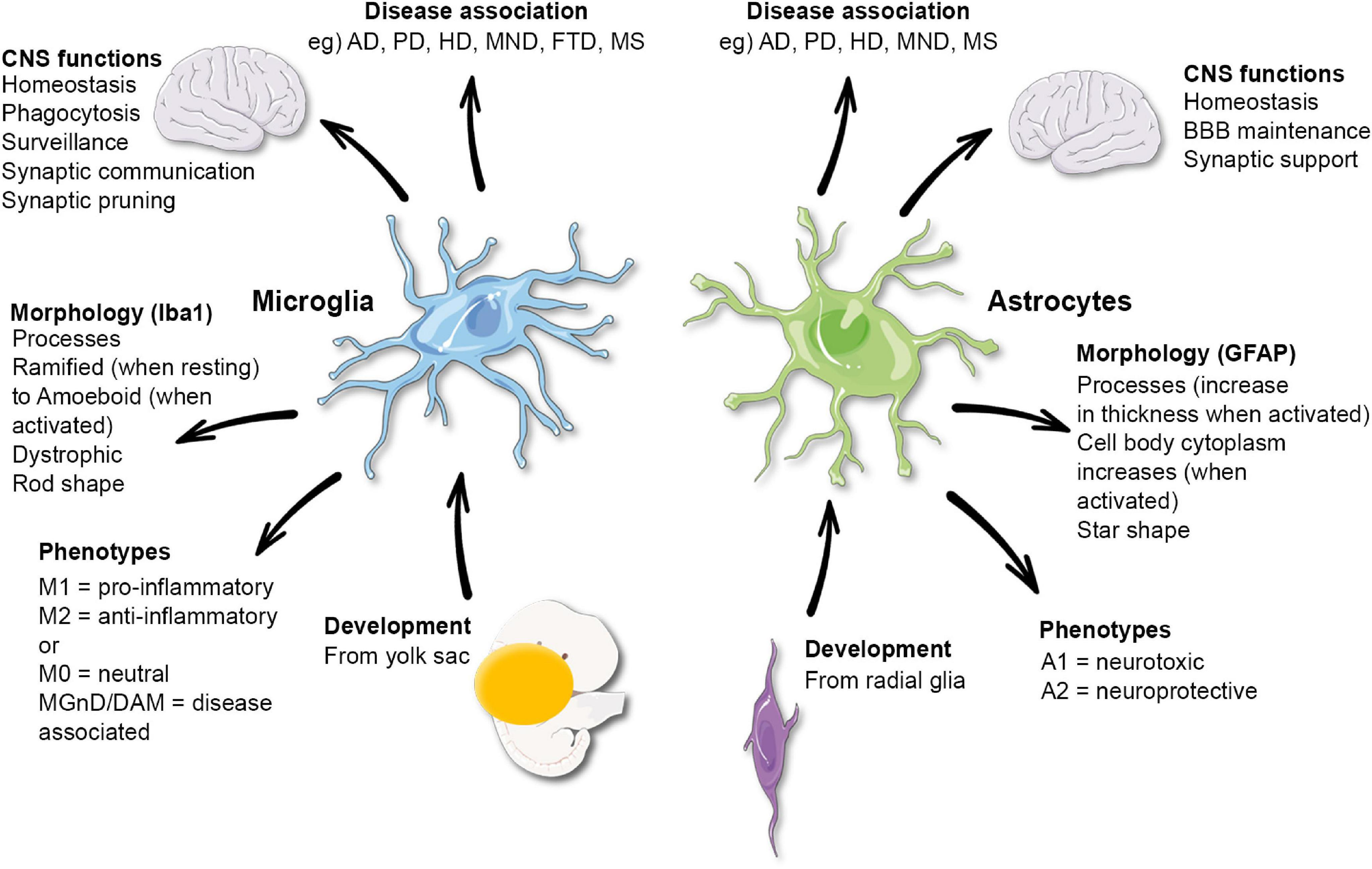
The Brain’s Immune System: Microglia at Work
Microglia serve as the brain’s primary immune defense, constantly monitoring the neural environment for signs of distress or damage. In performing their protective roles, they engage in critical processes like phagocytosis, where they engulf and digest cellular debris and pathogens. This activity is vital for maintaining homeostasis within the brain; however, when microglial function goes awry, it can lead to conditions such as neuroinflammation and synaptic dysfunction, which are hallmarks of diseases like Alzheimer’s.
Understanding how microglia operate under normal and pathological conditions provides insights into potential interventions for neurodegenerative diseases. Studies reveal that modulating the activity of these immune cells could prevent or mitigate the damage associated with Alzheimer’s disease. By enhancing the beneficial functions of microglia while inhibiting their detrimental effects, researchers may discover new therapeutic strategies that harness the brain’s immune system to combat Alzheimer’s and other related disorders.
Advancements in Neurodegenerative Disease Treatments
The landscape of treatment options for neurodegenerative diseases is evolving, driven by advancements in our understanding of underlying biological mechanisms. Research into the role of microglia in Alzheimer’s disease has been a significant contributor to this progress. Innovative therapies are being developed that involve targeting neuroinflammatory pathways, which may help slow or even reverse cognitive decline.
In addition to pharmacological approaches, emerging technologies like gene editing and cellular therapies hold promise for future treatments. These innovative strategies aim to restore normal microglial function or replace lost neuronal cells, offering hope to millions affected by neurodegenerative conditions. As scientific discoveries continue to unravel the complexities of diseases such as Alzheimer’s, the future of treatment looks increasingly promising.
Neuroscience Discoveries Revolutionizing Alzheimer’s Research
Recent discoveries in neuroscience are revolutionizing our understanding of Alzheimer’s disease and offering fresh perspectives on its treatment. Studies focusing on the brain’s immune system, particularly the function of microglia, have unearthed critical insights into how immune responses can influence neurodegeneration. This has shifted the paradigm from purely symptomatic treatment to exploring avenues that might intervene in the disease’s progression.
As research progresses, the integration of findings from different studies creates a comprehensive picture of how Alzheimer’s disease develops and how it can be addressed. By continually uncovering the connections between microglial activity and neurodegenerative processes, scientists are equipping themselves with the knowledge necessary to create targeted therapies that address the root causes of diseases rather than just their symptoms.
Federal Support in Alzheimer’s Disease Research
Federal funding has played a crucial role in advancing Alzheimer’s research, providing the necessary resources for scientists to explore innovative ideas. Agencies like the National Institutes of Health (NIH) have long supported critical research initiatives that explore the biological basis of neurodegenerative diseases. The backing from these institutions enables researchers to pursue ambitious projects that could lead to transformative scientific discoveries.
Beth Stevens’ work, particularly with microglial cells, exemplifies how foundational research funded by federal support can culminate in significant progress in understanding and treating Alzheimer’s disease. This investment in curiosity-driven science not only fosters a thriving research environment but also aids in the discovery of therapeutic avenues that could ultimately improve the lives of millions suffering from Alzheimer’s.
The Future of Alzheimer’s Disease Research
Looking ahead, the future of Alzheimer’s disease research appears promising, particularly through continued investment in innovative scientific approaches. As understanding of the complex interplay between microglial function, inflammation, and neurodegeneration deepens, researchers will be better equipped to develop novel therapeutic strategies aimed at Alzheimer’s disease. The integration of multidisciplinary studies is critical to achieving breakthroughs that can change the current treatment landscape.
Moreover, the application of advanced technologies such as machine learning and artificial intelligence in analyzing vast amounts of biomedical data will enhance research efficiency and efficacy. By facilitating the identification of potential biomarkers and therapeutic targets, these technologies will support the ongoing battle against Alzheimer’s and other neurodegenerative diseases, driving the field forward into new territories of possibility.
Understanding Neuroinflammation in Alzheimer’s Disease
Neuroinflammation is a common theme in Alzheimer’s disease research, as it plays a significant role in the progression of the disorder. This inflammatory response, mediated by microglial cells, can be both protective and detrimental. While microglia are essential for clearing away debris and damaged neurons, when their activity becomes dysregulated, it can lead to a chronic inflammatory state that exacerbates cognitive decline.
Current studies are focused on uncovering the balance of microglial responses in neuroinflammation, aiming to understand how to modulate these cells effectively as a therapeutic strategy. By focusing on the dual roles of microglia, researchers are investigating ways to enhance their beneficial functions while curbing their potential to drive neurodegeneration. Understanding the nuances of neuroinflammation is essential for developing targeted interventions for Alzheimer’s disease.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease?
Microglial cells serve as the brain’s immune system, continuously monitoring for signs of illness or injury. In Alzheimer’s disease, dysfunctional microglial activity can lead to aberrant synaptic pruning, which may contribute to the disease’s progression. Research shows that understanding microglial behavior is crucial for developing biomarkers and treatments for neurodegenerative diseases.
How are microglial cells related to neurodegenerative diseases?
Microglial cells are implicated in various neurodegenerative diseases, including Alzheimer’s disease and Huntington’s disease. They help maintain brain health by clearing dead cells and supporting neuron connections. However, abnormal microglial functions can exacerbate neurodegenerative processes, emphasizing their importance in current microglial research.
What scientific discoveries have been made regarding microglia and Alzheimer’s disease?
Recent scientific discoveries in neuroscience have revealed that microglial cells are key players in Alzheimer’s disease pathogenesis. Studies conducted by researchers, such as Beth Stevens, indicate that microglial dysregulation leads to inappropriate synaptic pruning, thus uncovering potential new biomarkers for Alzheimer’s and therapeutic targets.
Can microglial research lead to new biomarkers for Alzheimer’s?
Yes, ongoing microglial research is pivotal in identifying new biomarkers for Alzheimer’s disease. By understanding how microglial cells contribute to the disease’s mechanisms, scientists aim to develop more effective diagnostic tools and treatments, ultimately improving care for those affected.
Why is the study of microglia important for the brain immune system?
The study of microglia is essential to understanding the brain immune system because these cells are the first responders to brain injury and disease. Research into microglial functions and dysfunctions provides insights into how neuroinflammatory processes can affect conditions like Alzheimer’s disease, offering potential pathways for therapeutic intervention.
How does microglial research inform treatments for Alzheimer’s disease?
Microglial research informs treatment strategies for Alzheimer’s disease by revealing the role of these immune cells in disease progression. Insights gained from studying the mechanisms of microglial activity can lead to innovative therapeutic approaches and interventions aimed at mitigating neurodegeneration.
What are the implications of aberrant microglial pruning in Alzheimer’s?
Aberrant microglial pruning in Alzheimer’s disease can lead to loss of synaptic connections, contributing to cognitive decline. Understanding these mechanisms is crucial for developing treatments that restore proper microglial function and prevent the detrimental effects of neurodegeneration.
What funding supports microglial research related to Alzheimer’s?
Microglial research related to Alzheimer’s disease is significantly supported by federal funding, especially from the National Institutes of Health (NIH). This financial backing enables researchers, like those in Beth Stevens’ lab, to explore critical questions about the brain’s immune system and develop potential interventions for neurodegenerative diseases.
How has the understanding of microglia evolved in recent years?
The understanding of microglia has evolved significantly, from viewing them merely as support cells to recognizing their integral role in the brain’s immune responses and synaptic pruning processes. This shift in perspective has paved the way for novel insights into neurodegenerative diseases like Alzheimer’s and has sparked increased microglial research.
| Key Points | Details |
|---|---|
| Microglial Cells | Act as the brain’s immune system, clearing out dead cells and pruning synapses. |
| Role in Disease | Aberrant pruning by microglia is linked to Alzheimer’s, Huntington’s, and other neurodegenerative diseases. |
| Beth Stevens’ Research | Her work has established a foundation for identifying new biomarkers and treatments for neurodegenerative diseases. |
| Educational Background | Started research in early 2000s, driven by curiosity and NIH funding. |
| Scientific Importance | Research on basic science allows for significant discoveries and understanding of human diseases. |
| Federal Support | NIH and other federal funding were crucial in supporting her research from the start. |
Summary
Microglial research is essential in understanding brain health and neurodegenerative diseases such as Alzheimer’s. By elucidating the roles of microglial cells in pruning and immune responses, researchers like Beth Stevens are paving the way for innovative treatments. The significance of foundational and curiosity-driven science cannot be overstated, as it lays the groundwork for breakthroughs that can ultimately improve patient care and outcomes.