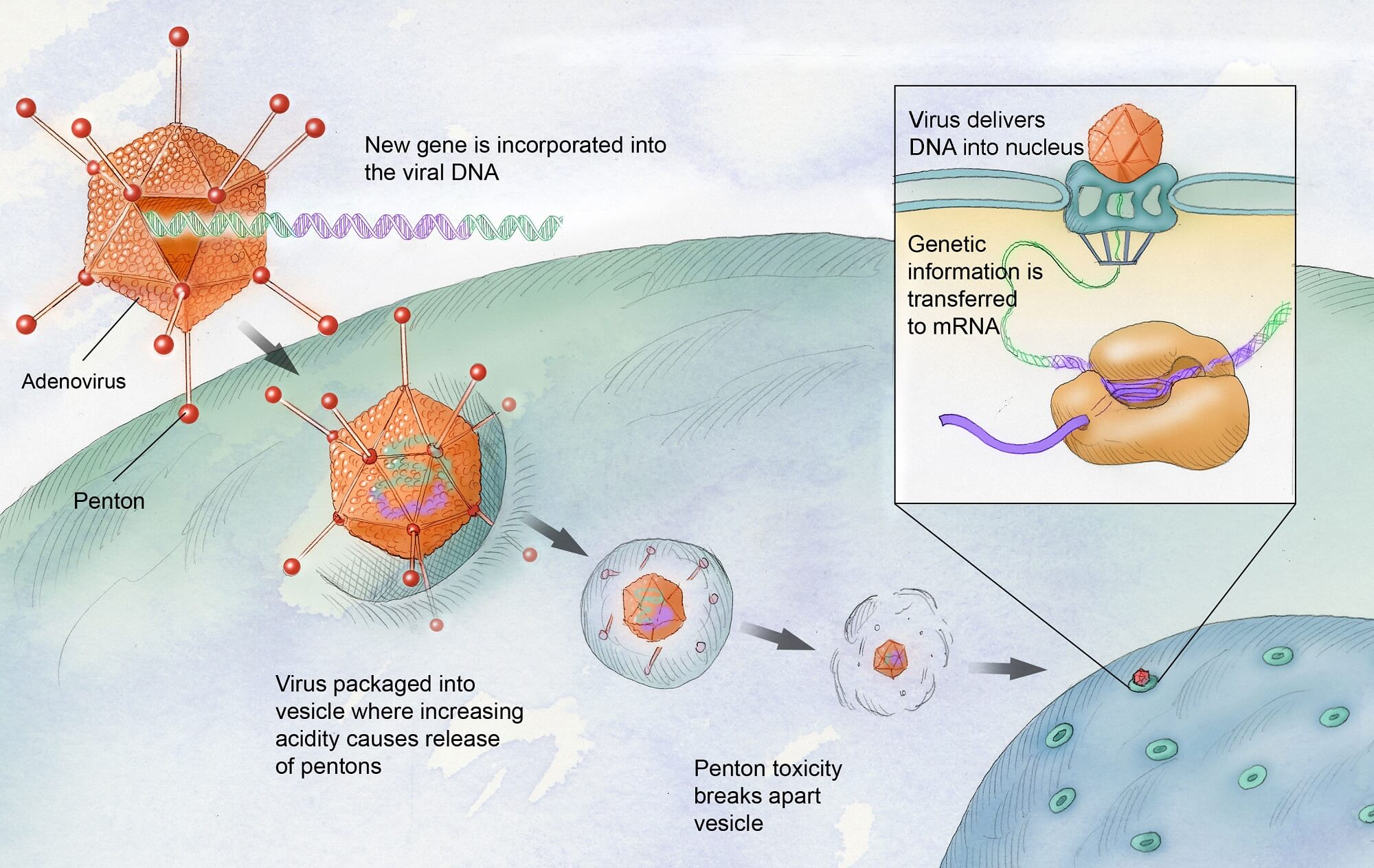
Gene therapy for hemophilia is revolutionizing the way we approach this genetic disorder, particularly in the case of hemophilia B. Patients like Terence Blue have experienced firsthand the transformative benefits of this innovative treatment, which aims to correct the underlying genetic defect responsible for missing clotting factors in the blood. Hemgenix, a groundbreaking therapy approved by the FDA in late 2022, exemplifies the potential of gene therapy to reduce dependence on traditional clotting factor therapy and eliminate the need for frequent injections. With advancements in medical science, the prospects for patients suffering from hemophilia treatment have never looked brighter. This pioneering approach not only offers hope but also promises a future where individuals can lead more active, worry-free lives without the constant fear of bleeding.
The emergence of innovative gene therapies marks a significant milestone in the treatment of bleeding disorders like hemophilia B. By harnessing cutting-edge genetic technologies, these novel treatments seek to address the root cause of the condition, providing patients with a much-needed alternative to conventional therapies. As gene therapies gain traction, they promise remarkable benefits, including sustained production of essential clotting factors and reduced administration of traditional factor therapies. The development of Hemgenix is a testament to this progress, demonstrating how the integration of advanced biopharmaceuticals is reshaping the landscape of hemophilia treatment. With a growing number of effective options on the horizon, patients are increasingly optimistic about managing their condition more effectively.
Understanding Gene Therapy for Hemophilia B
Gene therapy for hemophilia B represents a significant advancement in the treatment landscape for this serious bleeding disorder. Hemgenix, a groundbreaking gene therapy, offers a novel approach by aiming to correct the underlying genetic defect that causes hemophilia. Overwhelmingly, patients like Terence Blue are expressing hope as they transition from traditional clotting factor therapy to this innovative treatment method. By administering a corrected copy of the gene responsible for producing clotting factor IX, Hemgenix promises the potential of long-term relief from the constant need for injections and the risk of spontaneous bleeding episodes.
This transition signifies a move toward a more permanent solution, alleviating the challenges that patients face daily. Gene therapy’s ability to effectively reduce or eliminate the need for clotting factor infusions by promoting the body’s own production of this vital protein is undoubtedly a game changer. With a promising trajectory evidenced by trial data and patient outcomes, gene therapy for hemophilia B is setting a strong precedent for future treatments aimed at genetically rooted conditions, potentially revolutionizing how patients manage their health.
The Benefits of Gene Therapy: A Lifelong Change
The benefits of gene therapy extend far beyond just the cessation of needles; they promise a profound change in the quality of life for those affected by hemophilia B. For patients like Terence Blue, the prospect of living without the continuous worry of infusions is incredibly liberating. Patients can enjoy activities, travel, and engage in social settings with much less fear of potential bleeding episodes. This freedom can significantly improve mental health and overall well-being, fostering a return to normalcy that has previously seemed unattainable.
Moreover, gene therapy represents a move towards more efficient healthcare management. With fewer infusions needed, patients are likely to encounter lower overall treatment costs in the long run, despite the initial high price tags associated with these therapies. The success of Hemgenix not only opens doors for patients with hemophilia B but also sets a template for treating other genetic diseases with similar gene therapy approaches, broadening the horizon for future medical innovations and reinforcing the importance of investing in genetic research and development.
The Challenges Facing Gene Therapy Market Adoption
Despite the groundbreaking benefits associated with gene therapy for hemophilia B, challenges remain regarding market adoption and patient acceptance. The high costs of treatments, exemplified by Hemgenix’s eye-popping price tag, create barriers for many patients, as insurance coverage can be inconsistent and vary dramatically. These financial hurdles raise critical questions about the sustainability and accessibility of gene therapies, particularly when drugmakers face intense scrutiny over pricing and patient outcomes.
Additionally, there is a palpable fear among patients and healthcare providers surrounding the unknowns of new therapies. While clinical trials offer promising data, the long-term efficacy and safety of gene therapies remain under observation. This hesitation can dampen enthusiasm and lead to slower patient acceptance, as many individuals are understandably reluctant to transition from conventional therapies that have offered some level of control over their conditions. It is crucial for stakeholders in the healthcare ecosystem to address these concerns, emphasizing the transformative potential of gene therapy alongside transparent discussions about costs, risks, and expected benefits.
Personal Experiences: Transformative Impacts of Hemgenix
Patient stories like Terence Blue’s highlight the transformative impact gene therapy can have on the lives of those with hemophilia B. Many individuals have lived under the long shadow of this condition, fearing everything from everyday activities to the potential for severe medical emergencies. With innovative treatments like Hemgenix, patients are beginning to experience what life can look like without the incessant need for needles or the constant anxiety of bleeding episodes. These firsthand experiences provide invaluable insight into the actual effects of gene therapies on daily living.
As patient stories circulate, they not only inspire hope but also play a critical role in educating others about the possibilities of modern medicine. When patients share their journeys of healing, they help to demystify gene therapy, encouraging wider acceptance and discussion in both medical circles and communities. Transforming the narrative around living with hemophilia is paramount, and patients are at the forefront, proving that with cutting-edge science and care, a brighter future is possible.
Clotting Factor Therapy: The Traditional Approach
Clotting factor therapy has been the cornerstone of hemophilia management for decades, allowing patients to lead more stable lives despite their condition. This therapy involves regular infusions of factor IX or other clotting agents that the body lacks, providing a temporary fix to the symptoms associated with hemophilia B. While this approach has undoubtedly saved lives and improved quality of life, it comes with its own set of challenges, including adherence to frequent treatments and the psychological burden of living with a chronic condition.
For many patients, daily or bi-weekly injections become an arduous routine, often leading to physical breakdowns and emotional fatigue over time. Despite advancements in clotting factor therapy, such as longer-acting products that reduce the frequency of infusions, many individuals still yearn for a more definitive solution. With the emergence of gene therapy, like Hemgenix, there is renewed hope for a substantial improvement that may eventually replace or radically reduce the need for traditional clotting factor therapy.
Current Research and Future Directions
The landscape of hemophilia treatment is rapidly evolving, thanks in large part to ongoing research aimed at refining and expanding gene therapy techniques. Current studies are focused not only on enhancing the delivery mechanisms of these therapies but also on understanding the long-term effects and safety profiles. Researchers are exploring ways to broaden the applicability of gene therapies to more individuals, including those with varying genetic backgrounds and severities of hemophilia.
This research is crucial as it paves the way for potentially making gene therapy accessible to a wider range of patients, including those who may not respond well to current treatments. The future of hemophilia management lies in these investigational studies and trials, which could lead to advanced therapies that better align with patients’ needs. The more we understand about gene therapy mechanics, the more we can tailor treatments to optimize patient outcomes and quality of life.
The Role of Patient Advocacy in Gene Therapy Awareness
Patient advocacy plays a vital role in the acceptance and implementation of new treatment modalities, including gene therapy for hemophilia B. Organizations dedicated to hemophilia awareness are crucial in educating patients and healthcare providers about emerging therapies like Hemgenix. These advocates raise awareness not only about the scientific advancements but also about the emotional and social ramifications of living with hemophilia, which can sometimes overshadow the medical aspects of treatment.
Furthermore, advocacy groups serve as a bridge between patients and the medical community, ensuring that voices of those affected by hemophilia are heard in research and development discussions. By fostering dialogue, these organizations can influence the direction of future therapies, help shape policy regarding insurance coverage, and ultimately aid in making life-changing treatments more accessible to all who need them.
Evaluating the Long-term Efficacy of Gene Therapies
As gene therapy for hemophilia B, particularly Hemgenix, gains traction, researchers are focused on evaluating its long-term efficacy and sustainability. Clinical trials have shown promising results, with patients experiencing significant increases in their factor IX production levels; however, long-term data is necessary to determine how these therapies hold up over time. Assessing durability of response is critical to ensuring that patients receive lasting benefits from their treatments and can maintain a high quality of life without the burden of frequent injections.
Understanding the longevity of these therapies also impacts clinical practices and treatment plans moving forward. The experience of patients from initial treatment through subsequent years will help inform best practices, make refinements to treatment protocols, and guide ongoing research into potential adjustments or combinations with other therapies. This evolving data creates a foundation for better-informed decision-making for both patients and healthcare providers.
Ensuring Access to Gene Therapy for All Patients
Ensuring equitable access to gene therapy for hemophilia B is paramount as this innovative treatment becomes more widely available. With the high costs associated with Hemgenix and other gene therapies, ongoing discussions surrounding healthcare policy and insurance coverage are critical. Advocates for hemophilia patients stress the importance of negotiating fair pricing structures, educating insurers about the benefits of covering gene therapies, and pushing for legislation that supports broader access to these groundbreaking treatments.
Equity in access must be prioritized to make sure that all patients, regardless of their socioeconomic status, can benefit from advancements in gene therapy. As more compelling success stories emerge, it will be crucial for the healthcare community to come together in fostering a system that alleviates the financial burden and makes innovative therapies a reality for every patient living with hemophilia.
Frequently Asked Questions
What is Gene Therapy for Hemophilia and how does it work?
Gene therapy for hemophilia involves using a modified virus to deliver a correct copy of the gene responsible for producing clotting factor to the patient’s liver. This method aims to enable the liver to produce the clotting factor IX, which is deficient in hemophilia B patients, reducing their dependence on regular infusions of clotting factor therapy.
What are the benefits of using Hemgenix as a treatment for hemophilia?
Hemgenix offers several benefits for hemophilia treatment, including the potential to reduce or eliminate the need for regular clotting factor injections, leading to improved quality of life for patients. Because it requires only a single infusion to provide long-lasting effects, it significantly decreases the burden of daily management associated with hemophilia.
How effective is Hemgenix for hemophilia B patients?
Clinical trials show that Hemgenix is highly effective for hemophilia B patients, with 94% of treated individuals not requiring prophylactic factor IX infusions three years post-treatment. This makes it a promising approach to managing and potentially alleviating the symptoms of hemophilia.
What should patients expect during the administration of Hemgenix for hemophilia?
Patients can expect the administration of Hemgenix to take approximately two hours as an outpatient procedure. After receiving the therapy, they will be monitored for a few hours to ensure there are no immediate adverse reactions before being discharged.
Are there any side effects associated with gene therapy for hemophilia?
While Hemgenix generally has a favorable safety profile, some patients may experience side effects such as liver enzyme elevation, which is usually manageable with steroids and monitoring. Each patient’s response may vary, and ongoing evaluations are essential to ensure safety.
How does gene therapy for hemophilia compare to traditional clotting factor therapy?
Traditional clotting factor therapy involves regular infusions to manage hemophilia symptoms, which can be burdensome. In contrast, gene therapy like Hemgenix aims for long-term correction of the underlying genetic defect, potentially reducing or eliminating the need for ongoing treatments.
What new developments are emerging in gene therapy for hemophilia treatment?
New developments in gene therapy for hemophilia are emerging rapidly, with several therapies, including Hemgenix, receiving FDA approval. Ongoing research continues to explore new vectors, improved safety profiles, and additional targeted therapies to enhance treatment options for patients with hemophilia.
Who is a suitable candidate for Hemgenix gene therapy for hemophilia B?
Candidates for Hemgenix gene therapy typically include adults and adolescents with hemophilia B who have previously required regular infusions of factor IX. Each patient needs a thorough evaluation by a specialist to determine eligibility and discuss potential risks and benefits.
What are the long-term expectations after receiving Hemgenix for hemophilia treatment?
Long-term expectations after receiving Hemgenix may include sustained levels of factor IX production and a significant reduction in bleeding episodes. However, while the therapy shows lasting effects, continuous follow-up with a healthcare provider is necessary for monitoring and managing any potential issues.
| Key Aspect | Details |
|---|---|
| Terence Blue’s Experience | First patient in New England to receive Hemgenix gene therapy for hemophilia B on February 6, 2025. |
| Gene Therapy Overview | Hemgenix was developed by CSL Behring and is designed to correct mutations causing hemophilia B. |
| Potential Benefits | Aims to reduce or eliminate the need for regular clotting factor injections, allowing for a more normal life. |
| Market Challenges | High costs (e.g., $3.5 million for Hemgenix) and market acceptance necessitate patient awareness and insurance negotiation. |
| Risks and Considerations | Side effects include elevated liver enzymes; long-term effects and treatment efficacy still under evaluation. |
| Patient Changes Post-Therapy | Blue’s factor IX levels improved significantly after treatment, indicating a positive response to gene therapy. |
Summary
Gene therapy for hemophilia has shown great potential in transforming the lives of patients like Terence Blue, who recently underwent treatment with Hemgenix. This pioneering approach does not only aim to eliminate the need for lifelong injections but also provides hope for a more normal life, free from constant worry about bleeding episodes. While there are challenges surrounding costs and market acceptance, the promising results observed in clinical trials encourage further research and patient engagement in this innovative therapeutic avenue.