In a groundbreaking new study, TIM-3 Alzheimer’s treatment emerges as a promising avenue for combating Alzheimer’s disease, which affects millions globally. This innovative approach utilizes immune checkpoint therapy, a method previously successful in cancer treatment, to enhance cognitive function by targeting the TIM-3 gene. The research showcased that by inhibiting the TIM-3 molecule in microglia, the brain’s immune cells, researchers were able to promote the removal of amyloid plaques, which are detrimental to memory and cognition. The findings, published in a recent edition of Nature, not only demonstrate potential improvements in memory for mice subjected to this treatment but also offer hope for new therapeutic strategies against Alzheimer’s. As we delve deeper into the relationship between microglia and plaques, the potential for TIM-3 treatment could redefine how we approach neurodegenerative diseases.
Exploring alternative terms for TIM-3 Alzheimer’s treatment, we find a novel approach to tackle Alzheimer’s disease through immune modulation techniques, particularly immune checkpoint therapy. This groundbreaking strategy focuses on the TIM-3 gene, integral to regulating the immune response within the brain, influencing microglial activity in clearing harmful plaques that impair cognitive function. By understanding how these immune mechanisms can be harnessed to improve brain health, researchers are paving the way for innovative therapies. Such studies highlight the essential connection between brain immunity and neurodegeneration, opening doors to potentially transformative treatments that could alter the course of Alzheimer’s Disease. Overall, advancing our knowledge of TIM-3 and its role in Alzheimer’s may lead to new paradigms in preventing and treating this devastating illness.
Understanding TIM-3 in Alzheimer’s Treatment
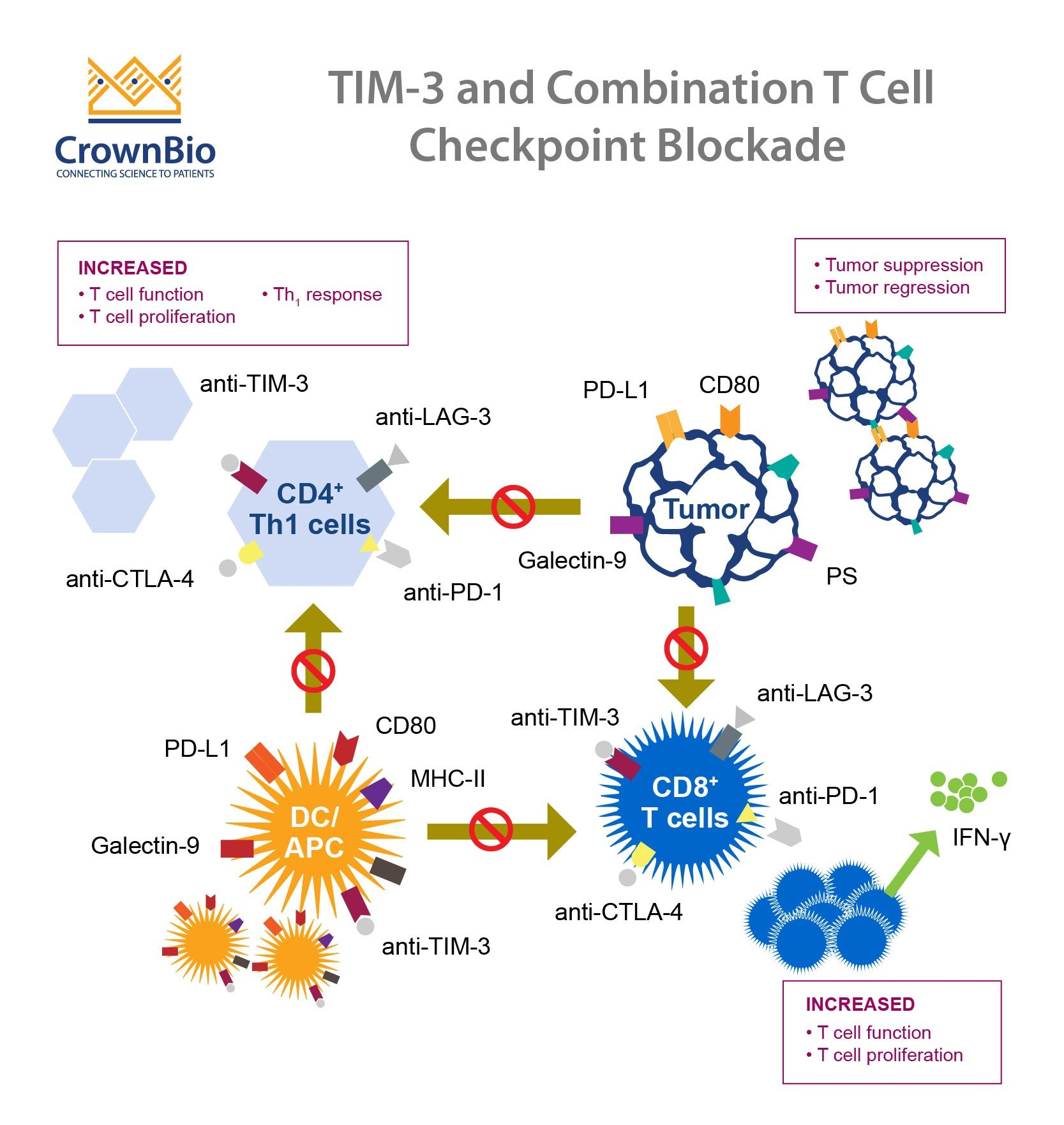
TIM-3, or T-cell immunoglobulin and mucin-domain containing-3, has emerged as a critical molecule in determining how the immune system interacts with Alzheimer’s disease pathology. It is a checkpoint protein that plays a vital role in regulating the activation of microglial cells, which are essential for clearing amyloid plaques from the brain. In Alzheimer’s patients, elevated levels of TIM-3 impede these immune cells from efficiently responding to plaque buildup, thus exacerbating cognitive decline. The study of TIM-3 in the context of Alzheimer’s treatment illuminates a promising pathway for therapeutic intervention, hinting at the potential to enhance memory and cognitive function by intervening in this inhibitory protein’s action.
Current research suggests that disabling the TIM-3 pathway could facilitate a more robust immune response against Alzheimer’s plaques. By genetically modifying mouse models to delete the TIM-3 gene, researchers have observed significant improvements in cognitive function and memory retention. This lines up with findings in cancers, where blocking TIM-3 has shown efficiency in revitalizing immune cell activity. With ongoing studies focused on anti-TIM-3 antibodies, there’s hope that similar therapeutic strategies could translate into effective treatments for Alzheimer’s disease, making TIM-3 a cornerstone for future research.
The Role of Microglia and Plaques in Alzheimer’s Disease
Microglia are specialized brain cells that act as the first line of defense in the central nervous system, akin to immune cells in the periphery. These cells are responsible for maintaining brain health, removing waste, and pruning synapses during development. However, in Alzheimer’s disease, microglia often become dysfunctional, failing to clear amyloid-beta plaques that are detrimental to cognitive function. The increasing expression of TIM-3 in these cells further complicates their role, leading to the accumulation of plaques and an overall decline in cognitive health. Understanding the delicate balance between microglial activity and plaque management is crucial for advancing Alzheimer’s treatments.
Recent findings have showcased the dual nature of microglia’s interaction with plaques in Alzheimer’s. On one hand, microglia are intended to protect neural health by engulfing and breaking down these harmful proteins. On the other hand, excessive activation of inhibitory molecules like TIM-3 hampers this critical function, allowing plaques to proliferate. Targeting this imbalance can reverse some of the neurodegenerative processes, highlighting the essential role of microglial response and TIM-3 regulation in steering Alzheimer’s research towards a viable treatment framework.
Innovations in Immune Checkpoint Therapy for Alzheimer’s
The application of immune checkpoint therapy in treating Alzheimer’s disease marks a groundbreaking innovation that draws parallels from successful cancer treatments. Immune checkpoint molecules, such as TIM-3, are primarily known for their role in preventing autoimmunity by masking overly activated immune responses. However, as seen in Alzheimer’s research, these molecules can be leveraged to optimize the brain’s healing processes. This approach not only aims to dissolve amyloid plaques through enhanced microglial activity but also reestablishes cognitive functions that are critical for patients suffering from this debilitating disease.
By employing checkpoint blockade strategies to alleviate TIM-3’s inhibitory effects, researchers aim to revitalize the natural immune response in the brain. This approach could transform the landscape of Alzheimer’s treatment by shifting the focus from solely targeting plaque reduction to enhancing the brain’s inherent ability to self-repair. As recent studies have illuminated pathways for developing anti-TIM-3 antibodies for clinical application, the potential to translate these findings into practical therapies presents an exciting frontier for both Alzheimer’s disease management and broader neurological health.
Cognitive Function Improvement through TIM-3 Intervention
The connection between TIM-3 intervention and improved cognitive function in Alzheimer’s has demonstrated promising results in preclinical models. Studies have shown that genetically modified mice lacking TIM-3 exhibited enhanced plaque clearance by microglia, leading to notable restorations in cognitive behaviors. Cognitive tests involving maze navigation and memory recall have provided vital evidence that reducing or blocking TIM-3’s effects can rejuvenate brain functions that are significantly impaired in Alzheimer’s patients. This breakthrough suggests a paradigm shift in understanding how immune modulation can directly influence cognitive health.
Moreover, the therapeutic implications of TIM-3 modulation extend far beyond simple plaque clearance. As demonstrated in laboratory settings, increased cognitive abilities in these models translated into improved emotional responses and behavioral outcomes, which are essential aspects of holistic health in Alzheimer’s patients. The potential to reclaim cognitive function not only enhances quality of life but could also pave the way for more comprehensive Alzheimer’s treatment strategies that encompass both the biological and psychological facets of the disease.
Next Steps in TIM-3 Alzheimer’s Research
As pioneering research explores the TIM-3 gene’s potential in Alzheimer’s treatment, several avenues are being actively pursued to determine the feasibility of human therapeutic applications. Researchers are currently focusing on the development and testing of anti-TIM-3 antibodies, aimed to selectively engage with the TIM-3 pathway to restore microglial functions. With the introduction of mouse models carrying the human TIM-3 gene, scientists are laying the groundwork for robust studies that could inform clinical trials, potentially leading to breakthroughs in how Alzheimer’s is treated.
In addition, ongoing investigations are assessing the cellular and molecular underpinnings of TIM-3 activity in microglia, aiming to reveal whether targeted manipulation can consistently yield positive outcomes in larger, more diverse populations. Future research will need to address the nuances in genetic variations and expression levels associated with TIM-3, particularly among different demographics affected by Alzheimer’s disease. This meticulous examination could launch the therapeutic application of TIM-3 towards a clinically validated approach, offering hope to millions grappling with this chronic illness.
The Genetic Landscape of Alzheimer’s Disease and TIM-3
Genetic factors significantly contribute to Alzheimer’s disease risk, with numerous studies emphasizing the TIM-3 gene’s association with late-onset forms of the illness. Polymorphisms in the HAVCR2 gene, which encodes TIM-3, have been linked to increased susceptibility for developing the disease. Identifying these genetic markers is crucial, as they could help predict which individuals are at higher risk for Alzheimer’s, allowing for earlier intervention strategies. Moreover, understanding the genetic landscape enhances the knowledge of how immune responses are altered in Alzheimer’s, providing deeper insights into potential treatment pathways.
Exploring the genetic variations related to TIM-3 could enable the development of personalized medicine approaches in Alzheimer’s treatment. Tailoring therapies based on individual genetic profiles allows clinicians to select the most effective strategies, enhancing the likelihood of positive outcomes. Furthermore, ongoing genomic studies pave the way for innovative research focused on the intersection between genetics and immunity, which could render groundbreaking discoveries that elevate TIM-3 from a simple genetic marker to a therapeutic target in the battle against Alzheimer’s disease.
Potential Risks and Considerations in TIM-3 Targeting
While targeting TIM-3 presents an exciting frontier for Alzheimer’s therapy, it is essential to acknowledge the associated risks and considerations. Immune checkpoint inhibitors like TIM-3 have the potential to disrupt homeostasis, leading to unintended autoimmune responses. As microglia play a dual role in neuroprotection and neurodegeneration, excessive activation could result in neuroinflammation, highlighting the need for careful calibration of TIM-3 therapies. The challenge lies in achieving the right balance—enhancing clearance of neurotoxic plaques without exacerbating immune-related complications.
Continued research is vital to understand the breadth of TIM-3’s impact on the immune system within the context of Alzheimer’s. Evaluating the long-term effects of TIM-3 modulation in clinical trials will help identify any adverse reactions that could arise as a result of altering immune checkpoint activity. This knowledge will ensure that therapeutic strategies are designed not only for efficacy but also for safety, ensuring that patients receive holistic care that minimizes risks while maximizing cognitive benefits.
Implementing TIM-3 Therapies in Clinical Settings
Transitioning TIM-3-targeting therapies from laboratory settings into clinical practice poses significant challenges and opportunities. Conducting well-designed clinical trials will be crucial in evaluating the efficacy and safety of anti-TIM-3 antibodies in Alzheimer’s patients. These trials need to carefully monitor cognitive outcomes, immune responses, and potential adverse effects to establish solid evidence for this innovative approach. Collaborations between researchers and healthcare practitioners will be instrumental in bridging the gap between scientific discovery and patient care.
Moreover, educating healthcare providers about the TIM-3 pathway and its implications for Alzheimer’s treatment will be paramount to successful implementation. As the medical community prepares for the potential rollout of TIM-3 therapies, stakeholders must engage in discussions about integrating these innovative solutions into existing Alzheimer’s treatment protocols. By advocating for research-driven approaches, the field can transform the current landscape of Alzheimer’s disease management, offering hope to patients and families alike.
The Future of Alzheimer’s Research and Treatment
The intersection of immunology and neurodegenerative diseases, particularly Alzheimer’s, signifies a new era of medical research and treatment development. The role of TIM-3 in mediating immune responses illustrates the potential of harnessing the body’s defense mechanisms for therapeutic advantage. As ongoing studies continue to unlock the complexities of TIM-3 and its effects on cognitive function, the future of Alzheimer’s treatment may lean heavily on immunomodulation strategies that empower microglial cells to perform their natural functions.
In conclusion, the potential of TIM-3 as a target in Alzheimer’s disease therapy holds great promise. As research progresses, integrating findings into practical applications will be crucial. With advancements in understanding immunological factors influencing Alzheimer’s, researchers and clinicians together can work towards innovative solutions that improve outcomes for those affected by this degenerative condition, instilling a renewed sense of hope in the fight against Alzheimer’s disease.
Frequently Asked Questions
What role does TIM-3 play in Alzheimer’s disease treatment?
TIM-3 is a checkpoint molecule that inhibits microglial cells in the brain. In Alzheimer’s disease, clearing amyloid plaques is crucial for improving cognitive function. By deleting TIM-3 expression, microglial cells can more effectively attack and remove these plaques, showing potential as a treatment strategy for Alzheimer’s.
How does TIM-3 affect microglia and plaque clearance in Alzheimer’s patients?
In Alzheimer’s patients, elevated levels of TIM-3 on microglia prevent them from clearing amyloid plaques that accumulate in the brain. This leads to a decline in cognitive function. Targeting TIM-3 could help restore the ability of microglia to remove these harmful plaques, potentially improving memory and overall brain health.
What are the implications of TIM-3 gene polymorphisms in Alzheimer’s disease?
Polymorphisms in the TIM-3 gene (HAVCR2) have been identified as genetic risk factors for late-onset Alzheimer’s disease. These variations can lead to increased TIM-3 expression, which compromises the ability of microglia to clear amyloid-beta plaques, thereby exacerbating cognitive decline associated with Alzheimer’s.
Can immune checkpoint therapy using TIM-3 be effective for Alzheimer’s treatment?
Yes, recent studies suggest that repurposing immune checkpoint therapy, which involves blocking TIM-3, may enhance the ability of microglia to remove amyloid plaques in Alzheimer’s patients. This approach has shown promise in animal models, indicating a potential therapeutic avenue for improving cognitive function in Alzheimer’s disease.
What experimental findings support the targeting of TIM-3 in Alzheimer’s disease?
Research has demonstrated that deleting TIM-3 from microglial cells in mice resulted in better plaque clearance and some restoration of cognitive functions. This supports the idea that TIM-3 is a significant barrier to effective immune responses against Alzheimer’s plaques, highlighting its potential as a therapeutic target.
What strategies are being explored for TIM-3 Alzheimer’s treatment?
Strategies include using anti-TIM-3 antibodies or small molecules that inhibit TIM-3’s function. By blocking this checkpoint molecule, the therapeutic approach aims to reactivate microglial clearance of amyloid plaques, offering a novel treatment pathway for Alzheimer’s disease.
Why is targeting TIM-3 considered a new direction in Alzheimer’s research?
Targeting TIM-3 represents a new direction in Alzheimer’s research because traditional treatments have focused on clearing amyloid plaques directly. By exploring the modulation of immune checkpoints like TIM-3, researchers aim to enhance the brain’s own defense mechanisms, providing a potentially more effective treatment for cognitive decline.
How does TIM-3 relate to late-onset Alzheimer’s disease?
TIM-3 has been linked to late-onset Alzheimer’s through genome-wide association studies. Its expression levels correlate with the presence of amyloid plaques in the brain, indicating that variations in the TIM-3 gene could influence the onset and progression of Alzheimer’s disease, making it a critical focus for potential therapies.
What are the next research steps for TIM-3 in Alzheimer’s treatment?
The next steps involve testing human anti-TIM-3 antibodies in Alzheimer’s mouse models to assess their effectiveness in reducing plaque accumulation. This ongoing research aims to translate findings from mice to potential treatments for humans affected by Alzheimer’s disease.
| Key Points |
|---|
| The immune checkpoint molecule TIM-3 may alleviate Alzheimer’s by enhancing microglial activity against plaques. |
| The majority of Alzheimer’s cases (90-95%) are late-onset, linked to the TIM-3 molecule. |
| Microglia are the primary immune cells in the brain, responsible for clearing plaques but inhibited by TIM-3. |
| Deleting TIM-3 in mice improved plaque clearance and cognitive function, demonstrating TIM-3’s role in inhibiting microglial action. |
| Potential treatment involves using antibodies or small molecules to block TIM-3, repurposing established cancer therapies. |
Summary
TIM-3 Alzheimer’s treatment presents a promising strategy for combating Alzheimer’s disease. Recent research demonstrates how targeting TIM-3 can potentially enhance the immune response against amyloid plaques, which are characteristic of Alzheimer’s pathology. This innovative approach, initially successful in cancer therapy, has opened new doors for Alzheimer’s treatment, signaling hope for improved cognitive function in affected individuals.